Translational Research Manager, Mike Gandy, and Dr Vanya Gant, Divisional Clinical Director for Infection at UCLH, explain the rise and rise of AI in pathology.

Mike Gandy

Dr Vanya Gant
Artificial Intelligence
The application of ‘artificial intelligence’ within pathology is gathering momentum and is the subject of considerable academic research and commercial interest. This not only comes from the traditional diagnostic and pharmaceutical sectors, but also from well known computing giants who, in recent years, have all entered the medical technology sector with various aims of utilising large clinical, demographic and/or image-based datasets to develop tools to support a wide range of clinical applications. The term artificial intelligence or AI is often now used as a catch all. In reality there is a range of different underlying technologies, ranging from relatively simple to complex systems, which can be deployed as tools within pathology in support of a clinical diagnosis.
Image analysis
There are already a number of image analysis systems at various stages of deployment across our pathology services.
They are based on predefined textural algorithms which utilise size, shape, density and colourimetric appearance to analyse and to segment cells or bacterial colonies and support the improved measurement, identification and interpretation of the material being analysed.
Although to cutting-edge technology organisations, these tools would seem old fashioned, their adoption in routine diagnostic practice is still a work in progress. Uptake over the past 15 years has been mixed, with the UK being one of the slowest of the developed nations to adopt this type of technology.
One of the key barriers to adoption of this type of technology relates, for some, to a reluctance to transition away from traditional light microscopy to analysis from a digital image.

APAS plate detecting MRSA colonies with typical target chromogenic intensity.
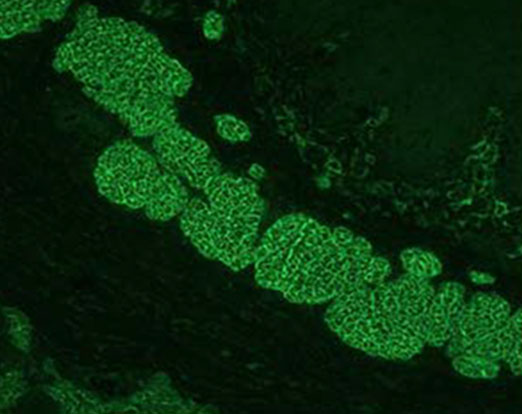
Aesku Helios results classification of positive staining of the muscularis mucosa of the endomysium.
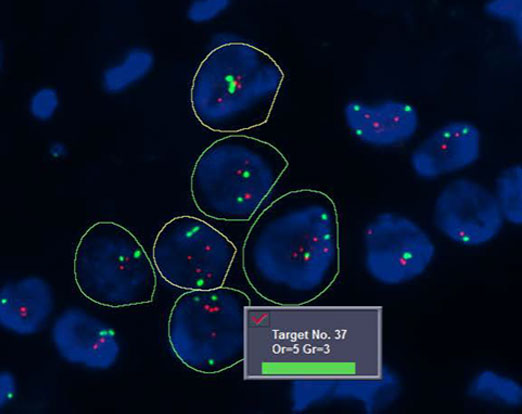
Bioview classification of HER2/neu gene (red) and centromeric chromosome 7 (green) with automated cell segmentation and target counting.
This is an area where we have seen some success within our own organisation - with digital offerings in use across Microbiology (Kiestra, APAS), Immunology (Aesku), Cytogenetics (CytoVision), Histology (Bioview) and Haematology (Sysmex).
Currently regulated by 510K (US) or CE (EU) level, these tools still generally rely on qualified operators selecting areas of interest and require abnormally detected patterns to be confirmed and authorised by a manual operator. They have initially found success in areas of quantification, replacing the need for manual cell counting or biomarker pattern recognition.
In some cases, image analysis algorithms are used to automatically screen out negative cell populations, leaving abnormal phenotypic patterns to be reviewed manually. These tools are particularly useful in high volume, low disease prevalence settings, where human factors involving repetitive examination of normal appearances in most cases may understandably come into play.
Machine learning
The healthcare landscape is changing with more complex decision support tools entering the diagnostic pathology arena. These tools, based on more complex machine learning protocols, require extensive, clinically annotated teaching and validation data sets to support the machine learning process.
Across pathology, these systems are being developed to support areas such as primary histopathological diagnosis by aiding pathologists in identifying areas of interest which show abnormal cellular architecture. In particular, we have seen this type of technology develop in areas where cancer shows a well-defined transition from normal, pre-malignant through to cancerous tissue.
This concept of AI-supported interpretation of diagnostic images also applies to radiology, where AI systems have begun to support decisions made by radiologists. The Sonic Healthcare global team have recently entered into an AI partnership with potential opportunities across radiology and pathology in the UK.
These multifactorial decision support systems are also being actively developed to address the growing need to distil the ever-increasing amount of genomic sequence data into results which will allow doctors to make better clinical decisions for their patients.
We fully expect to see these machine learning-supported results being deployed not only for better diagnoses but also for many other decisions, ranging from which cancer chemotherapies to give, to which antibiotics and antivirals will work in this age of increasing resistance.
How such AI systems best ‘sit’ alongside the laboratory equipment which generate large and complex (usually genetic sequence) datasets in the first place remains to be established, ranging from proposed ‘local, in-box’ solutions, to those delivered by cloud-based computing algorithms, and probably just about everything in between.
Cognitive computer based learning & neural networks
One area where advanced AI systems may be implemented first is in the case of the real-time management of advanced workflows within complex, multifactorial, ever-changing hospital environments.
Looking ahead at artificial intelligence in the context of systems with the ability to continuously learn, develop and improve their ‘diagnostic’ outputs with limited human intervention, these systems are very much still in their infancy.
The regulatory landscape is unclear for adaptive systems-based intelligence. These systems’ design is such that they ‘learn on the go’ and are capable of continuous improvement and applying those improvements to whatever they are designed to do, without a systems wide validation or ‘approval’ checkpoint.
This also implies that as they ‘learn’ from their local environment (of patient- and pathogen-related data), they also learn about this local environment.
It implies that further benefit may flow from their ‘local’ knowledge, thereby creating a tool which is unique to the setting in which it is deployed. After all, this is what we as humans do every day. We layer cognitive reasoning alongside overarching clinical guidance to locally based problems specific to our own organisation, generating an often unique solution.
Advanced AI systems may also offer benefits beyond the laboratory in the case of the real-time management of advanced workflows within complex, multifactorial, ever-changing hospital environments. These solutions will be designed to support the need to rapidly gain patient flow efficiencies within a hospital.
It remains to be seen to what degree these systems are able to effectively and usefully process multifactorial internal and external information streams. These range beyond those specific to laboratories, to diverse clinical activities, from ambulatory pathways, emergency room availability, surgical and other treatment planning, therapeutics and discharge processes, to public transport, and even triggering public health alerts.
Exciting times ahead
Machine learning and AI-based systems are here to stay. They offer huge potential for making better decisions, be they diagnostic or clinical, learning from millions (in some cases) of other data points, in thousands of different clinical and diagnostics spaces.
It’s truly a very exciting time to be working in pathology and to be on the front line as Sonic Healthcare UK writes the next chapter of AI and machine learning for our future diagnostics.