HSL Advanced Diagnostics specimens
Guidance on slides, tissue placement, and slide drying/baking.

Slide requirements
All sections cut for immunohistochemistry (IHC) or fluorescent in situ hybridisation (FISH) testing require special precautions for optimal performance and quality of staining procedures. Sections should be cut onto the recommended slide type.
Immunohistochemistry (IHC) – stain and return only
For stain and return IHC requests, we require sections cut at 3-4μm placed on positively charged IHC slides. Please provide an appropriate number of unstained sections to cover the number of requests per case, plus an additional 2–4 sections for repeat staining that may be required.
Special stain (stain and return)
For special stains, most sections can be cut at 3μm onto positively charged IHC slides. Please provide an appropriate number of unstained sections to cover the number of requests per case. See the tables on pages 6 and 7 for slide requirements for Reticulin stain.
IHC and FISH for interpretation
For all interpretative requests, we require an appropriate number of unstained sections plus an additional 2 unstained sections for repeat/reflex testing that may be required.
Tissue placement
Routine Stain and Return IHC, MMR
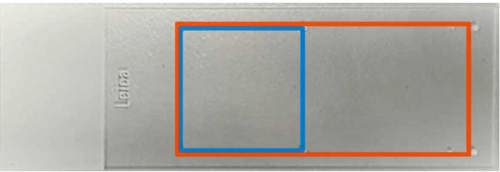
Section placement should not be excessively high. HSL-AD primarily uses the Leica Bond III platform for IHC staining and the staining area does not cover the entire surface area of the slide.
- Sections from biopsies and small pieces of tissue should be placed in the area within the bluebox.
- Sections from resections and larger / multiple pieces of tissue should be placed in the area covered by the red box.
- Placement of tissue in this way is applicable for all slide types.
- It is very important that the lesion of interest from mega blocks is placed on the test slides appropriately.
Her-2, ER, ALK, ROS1, p16, pan-TRK, PD-L1 and FISH Tests
These IHC tests are primarily performed on the Roche / Ventana and Agilent / Dako platforms.
- Section placement should be in the top third of the slide.
- Sections from resections and larger / multiple pieces of tissue should be placed as required, leaving some free space towards the bottom of the slide.

Virology / Bacteriology IHC Requests
- Cut a ribbon of 3 sections on each slide referred for the these tests e.g. CMV, H. pylori, T. pallidum [Syphilis] and VZV.

Slide drying / baking
- Once cut, all sections for IHC and FISH should be left to dry naturally or in a slide rack above a gentle heat source in an upright position for 30 minutes to 1 hour.
- Ensure that there is no remaining water underneath the section before baking. The use of slides recommended in this user guide are selected to optimise the drying process, reduce the time required and significantly reduce the number of repeat tests we perform.
- All sections for IHC or FISH should be placed in a temperature controlled oven at 60oC for 1 hour or at 37oC overnight.
- Sections should not be hot-plated using direct heat on the slide, as this may cause poor tissue adherence and unreliable IHC / FISH staining quality.
- X-tra® Slides, from any manufacturer, are not suitable for FISH testing.
- If slide identification at the referring laboratory is done through printed labels, the maximum height of these labels must not exceed 22mm. If this is not possible, slides handwritten with pencil are preferable. This is critical as HSL-AD primarily uses a range of staining platforms. IHC/CISH staining on these have been calibrated and validated for a specific slide surface area. Furthermore, labels extending down onto the slide staining area may exert a hydrophobic effect on the reagents applied.
- Any deviations to these instructions may lead to compromised staining quality.